Got data?
Quantiative Engineering Design (QED.ai) overcomes data gaps in the developing world by building data systems that are practical and sustainable.
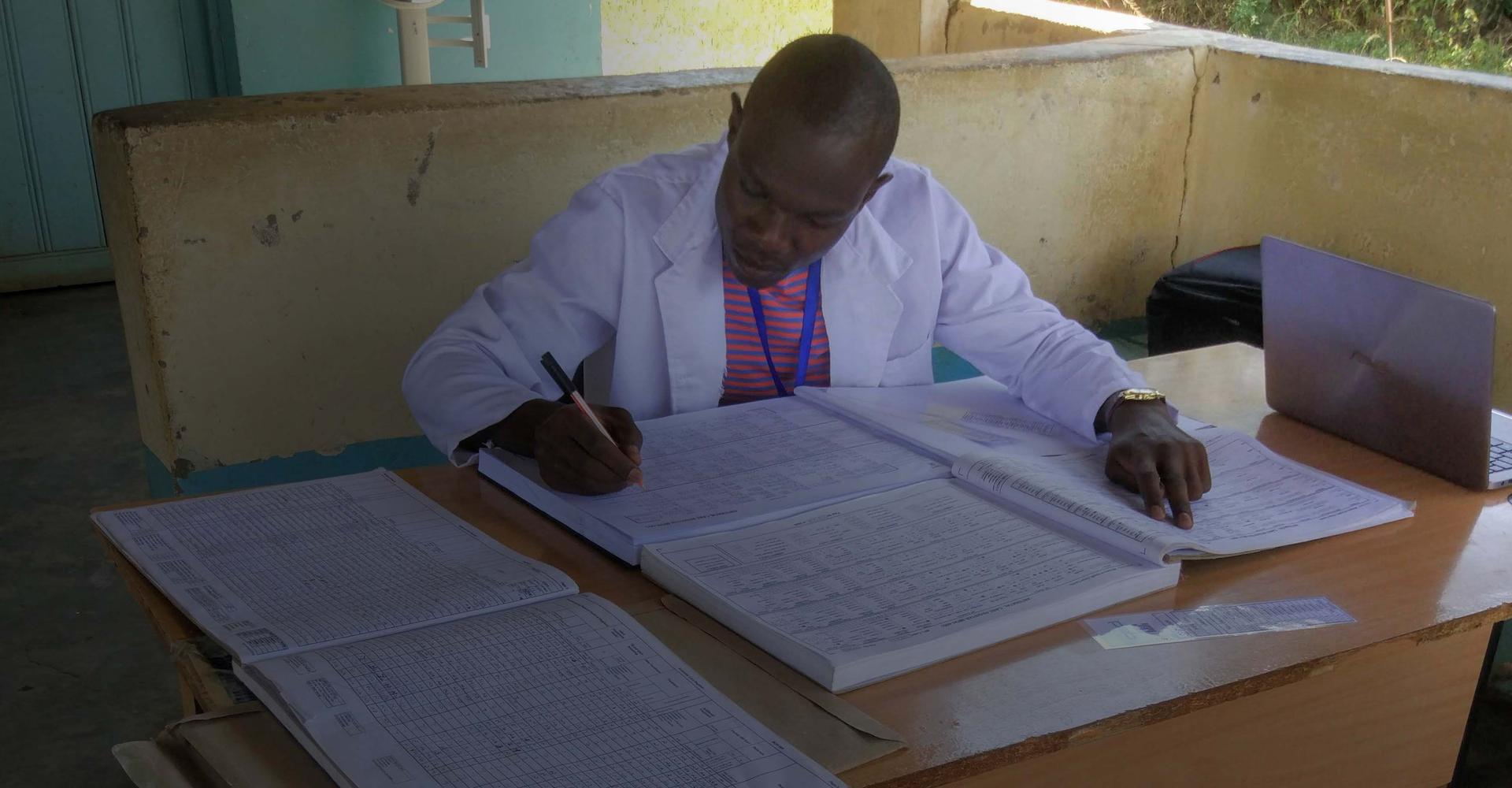
Gather data in challenging environments
ScanForm
Scannable form technology that bursts the efficiency of using paper forms by rapidly digitizing handwritten contents into Excel, in under 60 seconds. All that is needed is regular paper, a pen, and an Android phone! Custom analytics are produced in real time.
Explore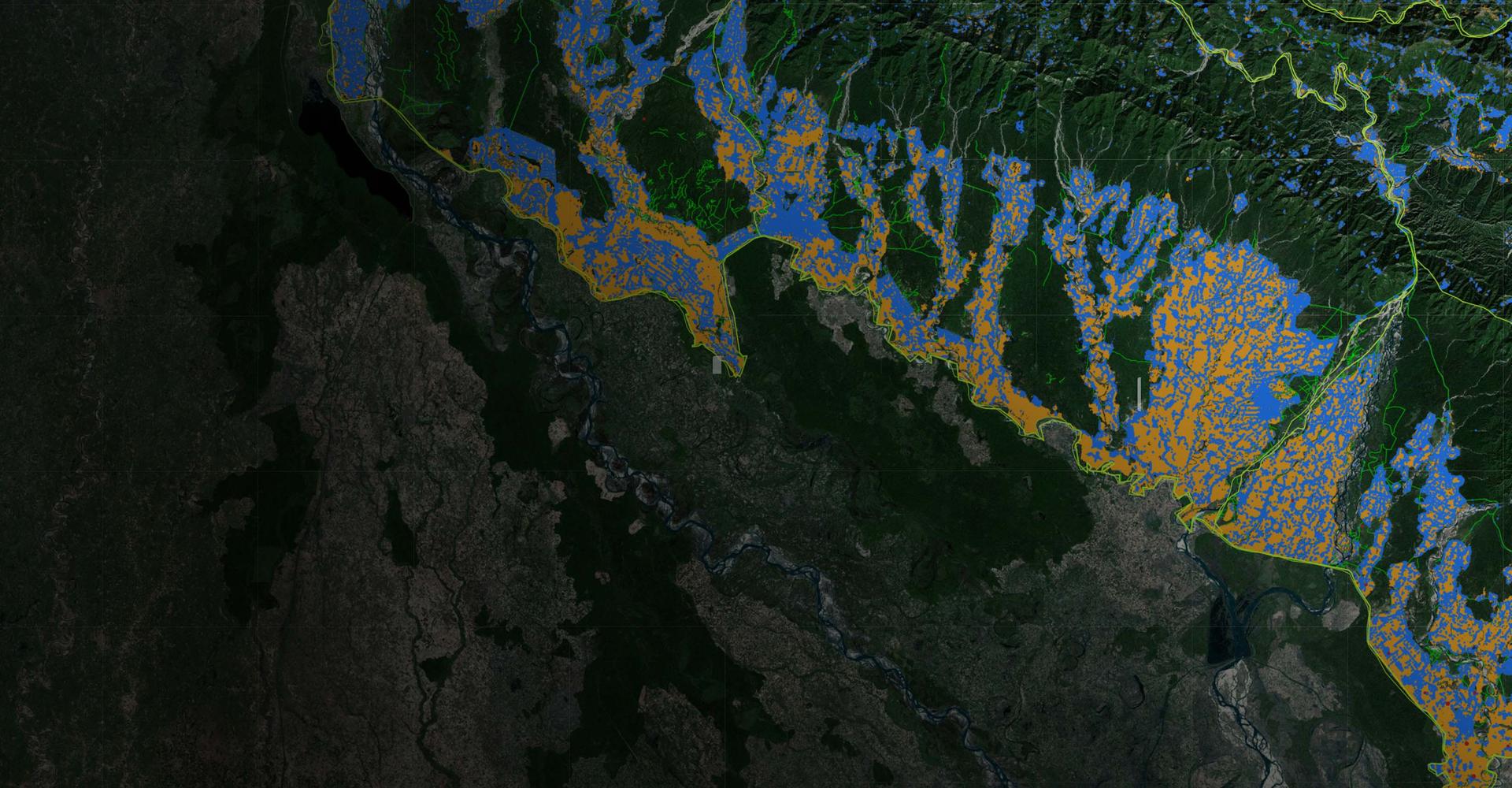
Geospatial mapping
Maps
Remote sensing and artificial intelligence enabling governments, donors, and the private sector to classify land-use at regional and national levels, focusing on Sub-Saharan Africa and South Asia.
Explore
Handheld spectroscopy
ScanSpectrum
A rugged and reliable handheld visible/near-infrared spectrometer (400-1000 nm), suited to field measurements of plants and for the environmental sciences.
Explore
portable automated carbon kit
PACK
Healthy soil is the foundation of a well-functioning landscape, and organic matter is key to healthy soil. PACK is a low-cost lunchbox-sized device used to measure soil carbon in the field.
Explore
Since 2012 we have provided mission-critical support to health and agriculture projects in 13 countries. QED helps its partners identify strengths, weaknesses, and scale up operations using software and AI. Our forte is leveraging, adjusting, and deploying technologies in highly demanding environments.
Learn more about QED
Where we have worked
Benin

Burkina Faso

Cambodia

Ethiopia

Ghana

India

Kenya

Malawi

Mexico

Mongolia

Namibia

Nepal

Nigeria

Slovakia

South Africa

Taiwan

Tanzania

Uganda

We work on projects globally, but our offices are located here
Kenya

Malawi

Poland

USA

Taiwan

FEATURED ON


Sometimes all it takes to make a difference is the willingness to learn about a problem and use your talents to help solve it.
Bill Gates, GatesNotes
Our Partners